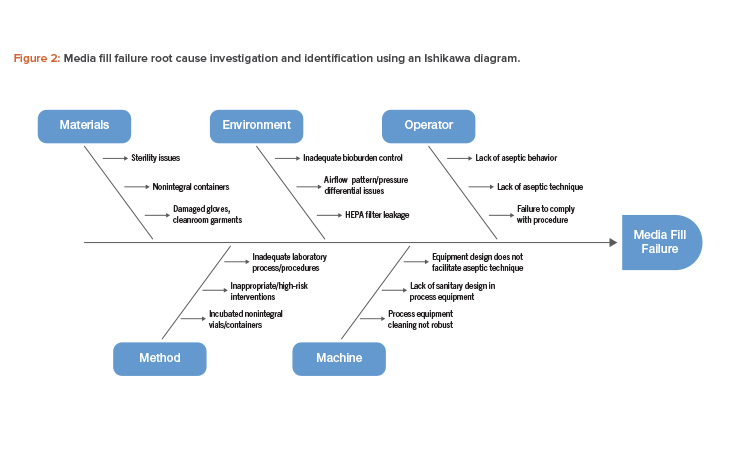
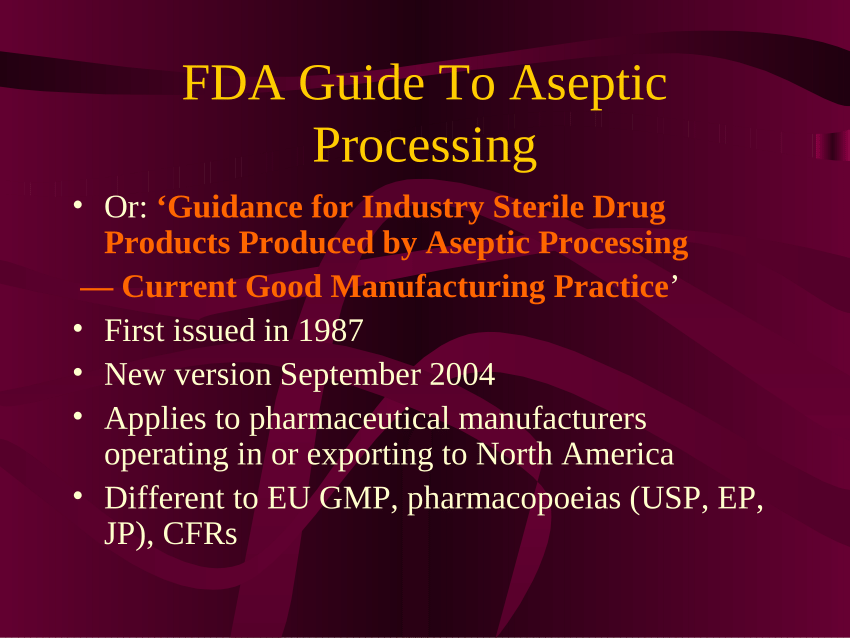
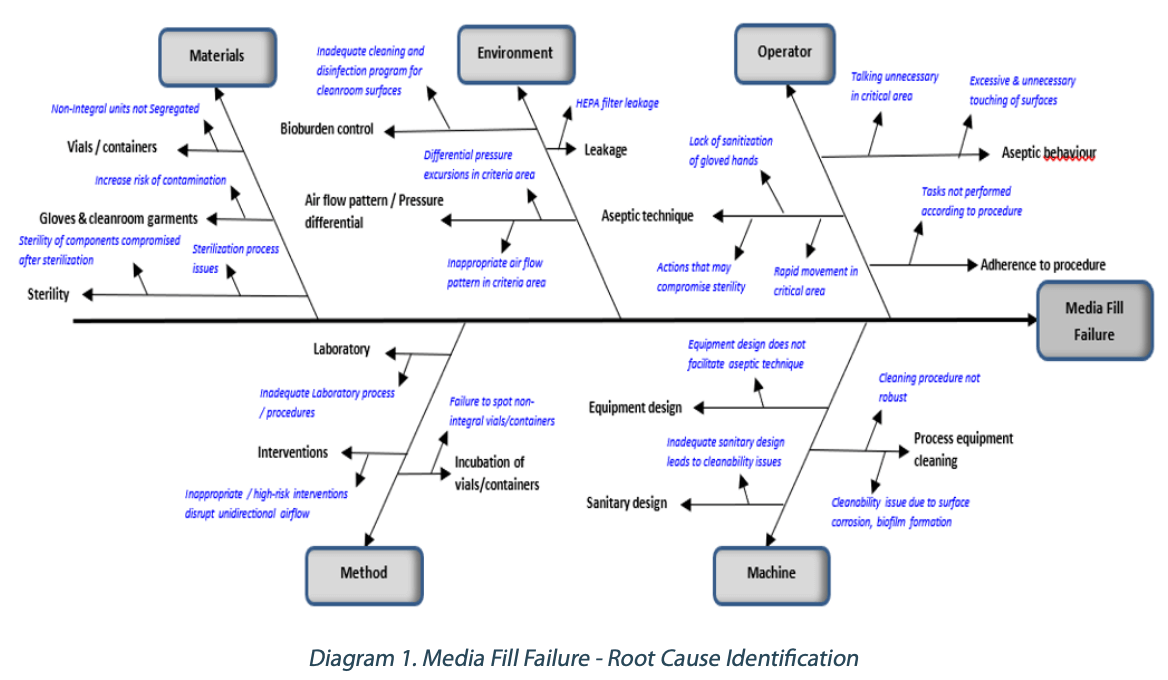
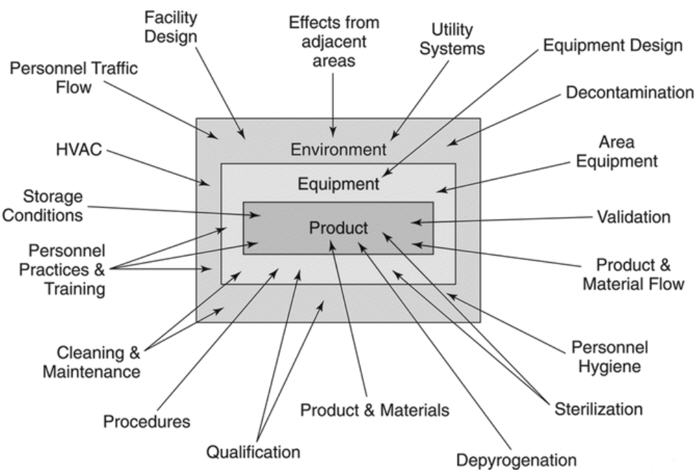
Guidance for Industry #253 - Good Manufacturing Practices for Animal Cells, Tissues, and Cell- and Tissue-Based Products

Guidance for Industry: Preparation of Premarket Submissions for Food Contact Substances (Chemistry Recommendations) | FDA