
A metal M of atomic weight 54.9 has a density of 7.42 g cm^(-3). Calculate the volume occupied and the radius of the atom of this metal assuming it to be sphere.
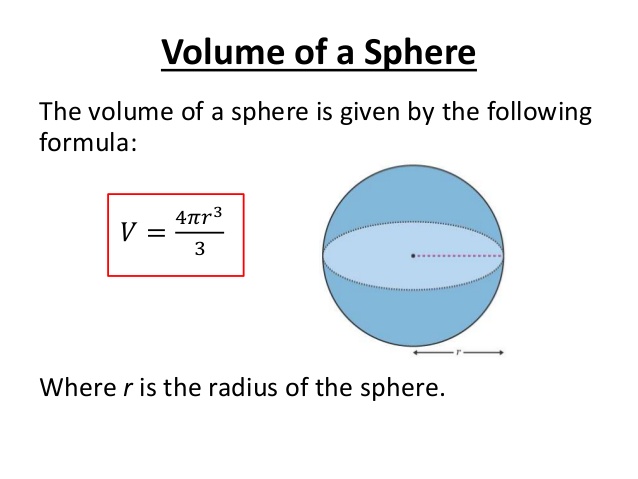
Atomic radius is of order 10^-8 cm and nuclear radius is of order 10^-13. calculate what fraction of atom is occupied by nucleus? | Socratic
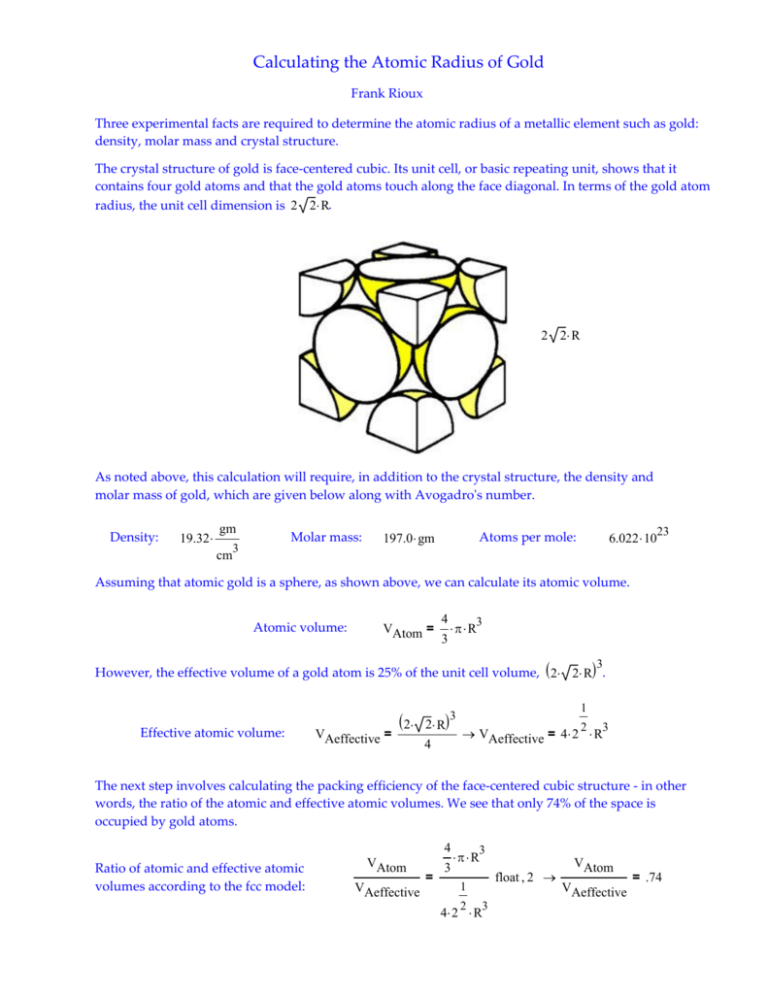
What is Atomic Packing Factor (and How to Calculate it for SC, BCC, FCC, and HCP)? – Materials Science & Engineering
Welcome to Chem Zipper.com......: How to calculate percentage (%) ionic character in covalent compounds?
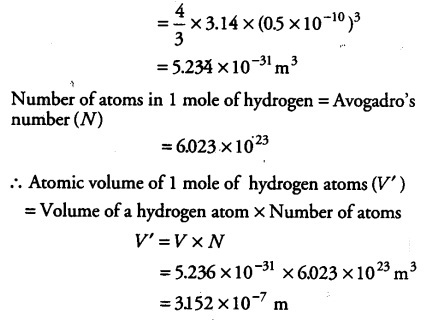



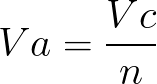
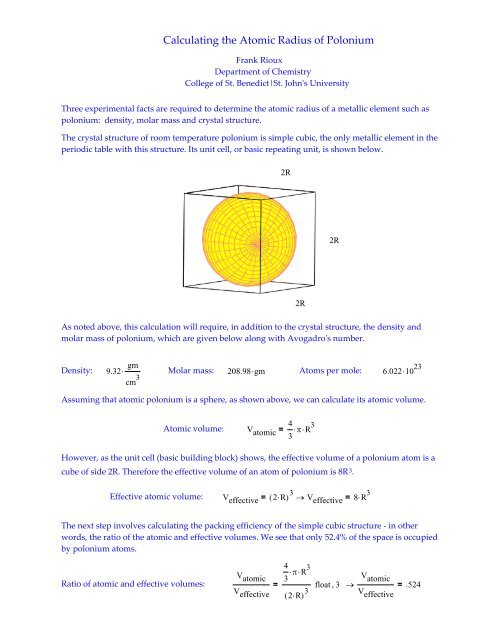


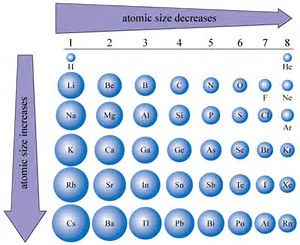

:max_bytes(150000):strip_icc()/nucleus-and-atoms-713783177-5a2bf9699e9427003730790b.jpg)



