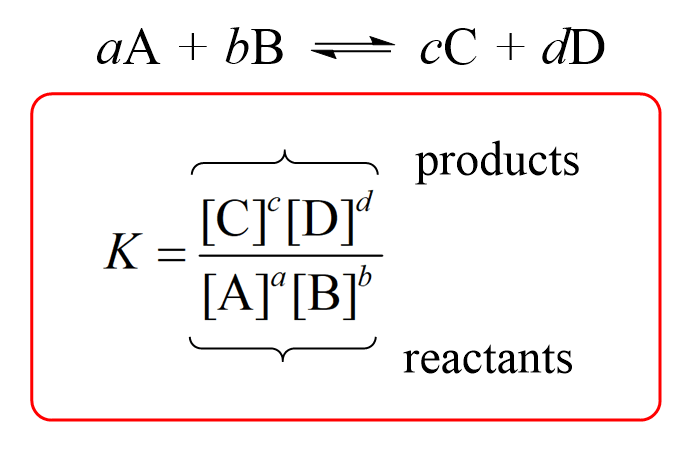
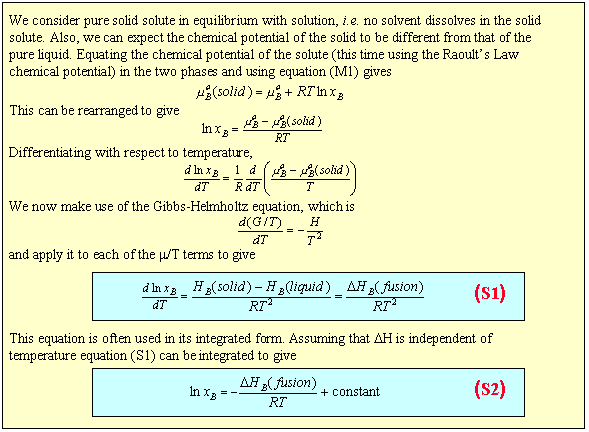
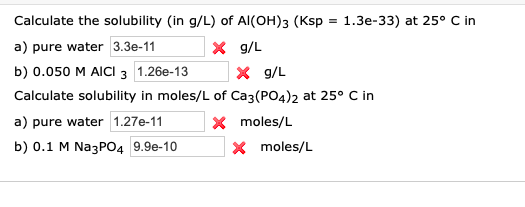
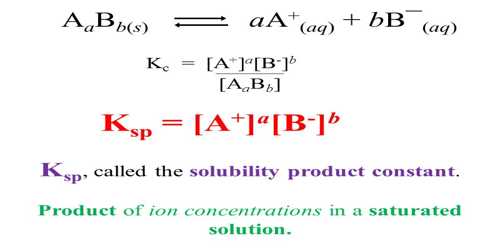
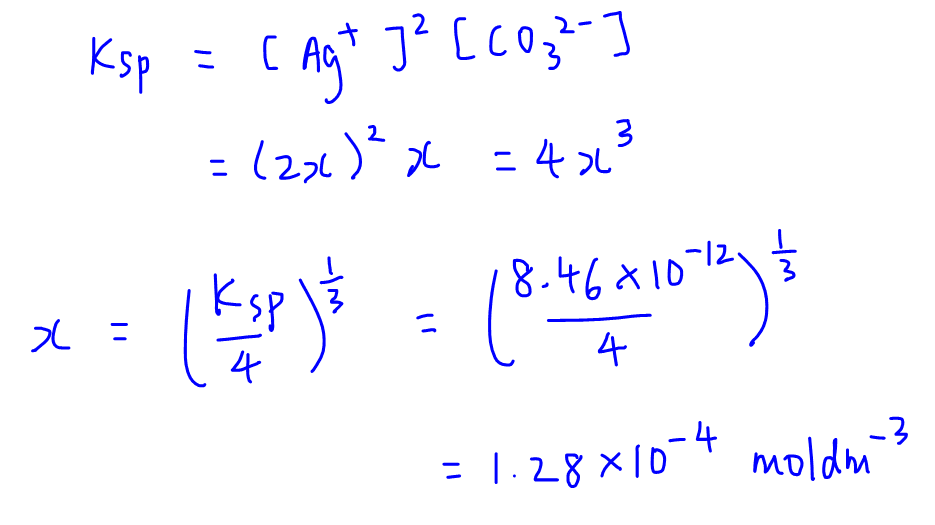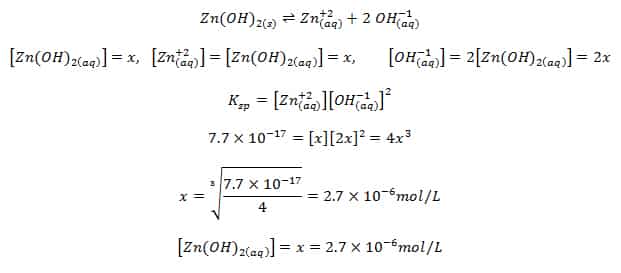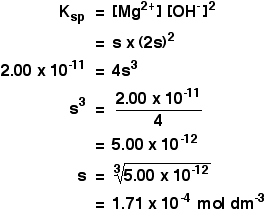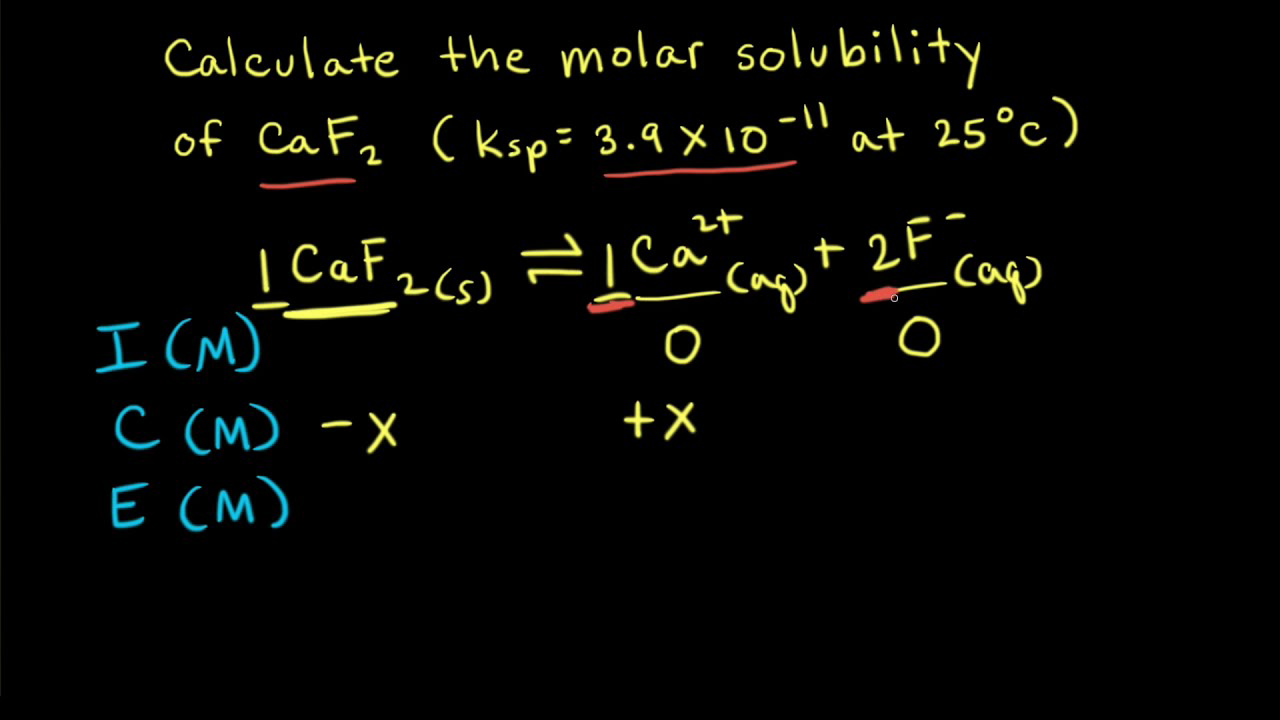Calculate solubility (in moles / litre) of a saturated aqueous solution of Ag3PO4 if the vapour pressure of the solution becomes 750 torr at 373 K(Assume molality = molarity).
Calculate the solubility product and solubility of AgCl in the following cell which has an emf of 0.455 volts at 25°C. - Sarthaks eConnect | Largest Online Education Community
What is the solubility of Pb(OH)2 in a buffer solution of pH8 if its solubility in water is 6.7*10^-6? - Quora

The calculated values of solubility parameters of polymers and solvents. | Download Scientific Diagram

Calculating the Equilibrium Constant from the given Solubility (Molarity) of a solution | Chemistry classroom, Ap chemistry, Chemistry
![Lesson 4 Calculating Solubility. 1.Calculate the 25 o C for BaCrO 4 in units of g/L. BaCrO 4(s) ⇌ Ba 2+ +CrO 4 2- ssssss Ksp=[Ba 2+ ][CrO. - ppt download Lesson 4 Calculating Solubility. 1.Calculate the 25 o C for BaCrO 4 in units of g/L. BaCrO 4(s) ⇌ Ba 2+ +CrO 4 2- ssssss Ksp=[Ba 2+ ][CrO. - ppt download](https://images.slideplayer.com/27/9060864/slides/slide_3.jpg)