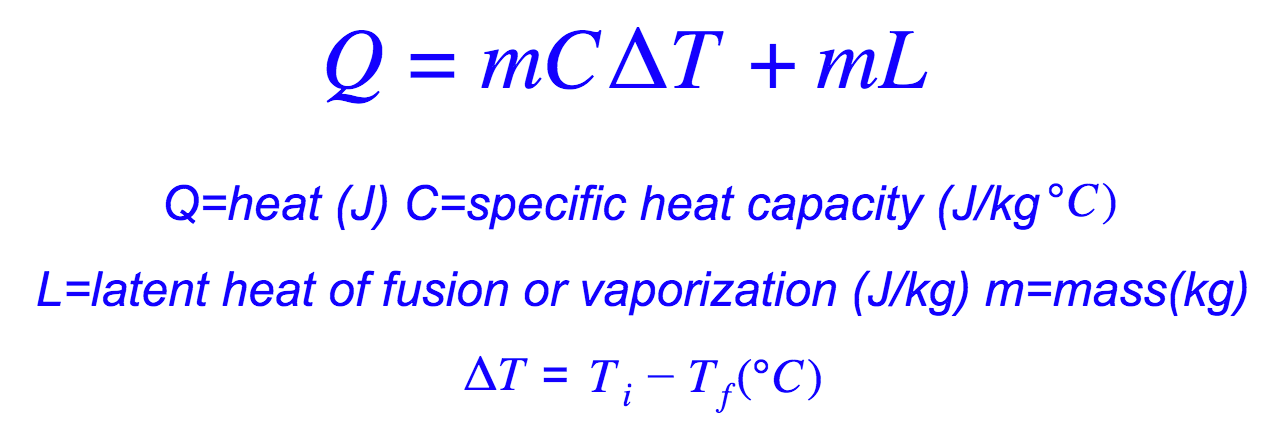Calculate the enthalpy of vaporisation per mole for ethanol. Given, Δ S = 109.8JK^-1mol^-1 and boiling point of ethanol is 78.5^oC .

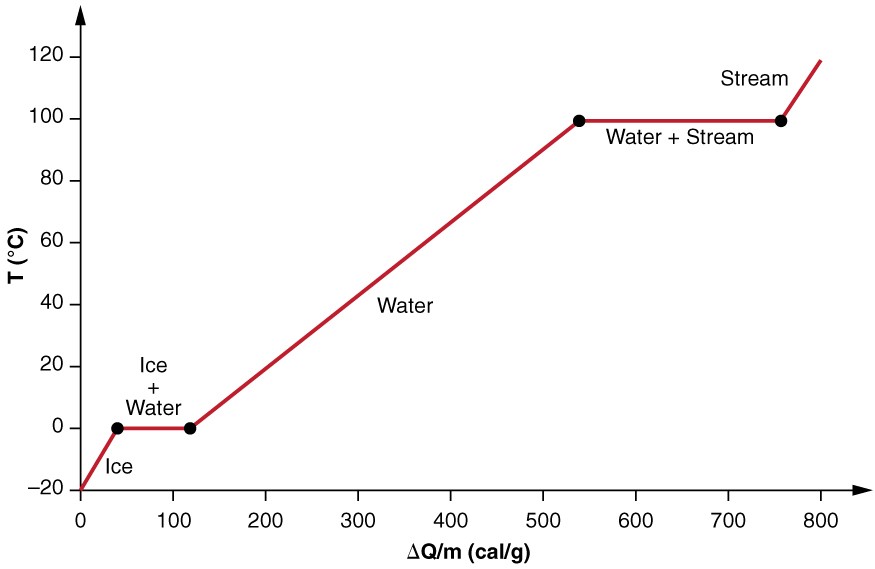
Calculate the heat required to convert 3 kg of ice at - 12^o C kept in a calorimeter to steam at 100^o at atmospheric pressure. (Given: specific heat of ice = 2.100 ×

Using Heat of Fusion or Vaporization to Find the Heat Needed to Melt or Boil a Substance | Chemistry | Study.com
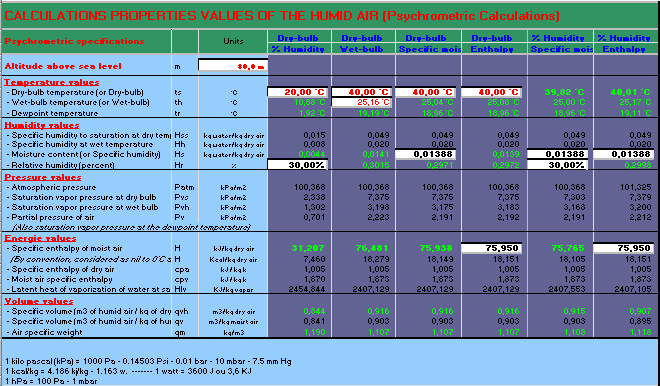
Misting, Evaporative, cooling, fogging, nozzles, temperature, humidity, moiture, dew, excel, calculation

Calculate the heat required to convert 3 kg of ice at - 12^o C kept in a calorimeter to steam at 100^o at atmospheric pressure. (Given: specific heat of ice = 2.100 ×





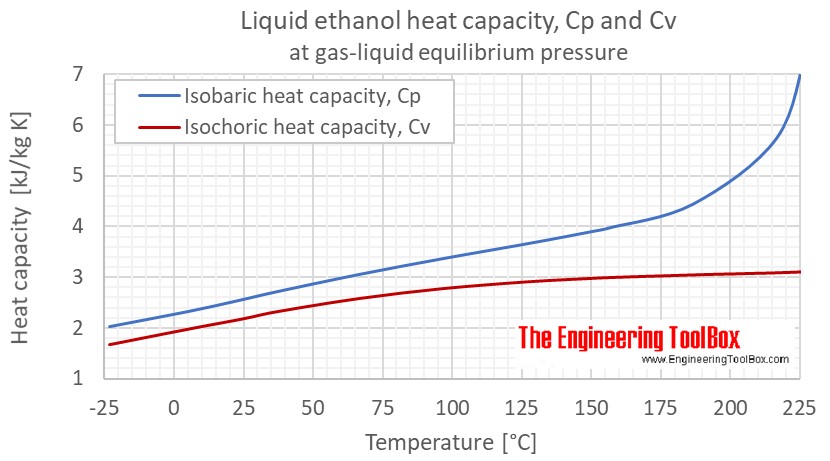

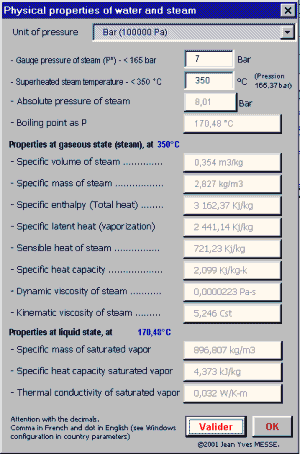
![Latent heat of vaporization for main components of LNG [10]. | Download Table Latent heat of vaporization for main components of LNG [10]. | Download Table](https://www.researchgate.net/publication/330572654/figure/tbl3/AS:718422421803010@1548296661881/Latent-heat-of-vaporization-for-main-components-of-LNG-10.png)